-
The information on this page is current as of April 1 2020.
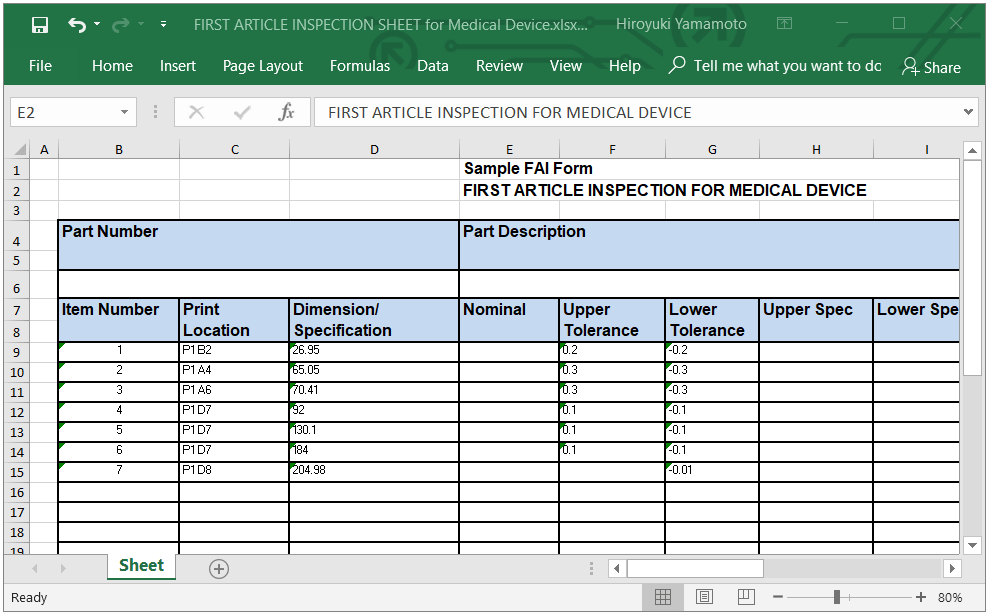
For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).

21 Cfr Part 820 Pdf Download
| | [Code of Federal Regulations] | | [Title 21, Volume 8] | | [Revised as of April 1, 2020] | | [CITE: 21CFR820.50] |
|
| CHAPTER I--FOOD AND DRUG ADMINISTRATION
DEPARTMENT OF HEALTH AND HUMAN SERVICES |
| | SUBCHAPTER H - MEDICAL DEVICES |
|
Subpart E - Purchasing Controls | Sec. 820.50 Purchasing controls. |
Each manufacturer shall establish and maintain procedures to ensure that all purchased or otherwise received product and services conform to specified requirements. (a) Evaluation of suppliers, contractors, and consultants. Each manufacturer shall establish and maintain the requirements, including quality requirements, that must be met by suppliers, contractors, and consultants. Each manufacturer shall: (1) Evaluate and select potential suppliers, contractors, and consultants on the basis of their ability to meet specified requirements, including quality requirements. The evaluation shall be documented. (2) Define the type and extent of control to be exercised over the product, services, suppliers, contractors, and consultants, based on the evaluation results. (3) Establish and maintain records of acceptable suppliers, contractors, and consultants. (b) Purchasing data. Each manufacturer shall establish and maintain data that clearly describe or reference the specified requirements, including quality requirements, for purchased or otherwise received product and services. Purchasing documents shall include, where possible, an agreement that the suppliers, contractors, and consultants agree to notify the manufacturer of changes in the product or service so that manufacturers may determine whether the changes may affect the quality of a finished device. Purchasing data shall be approved in accordance with § 820.40. |
|
|
View Title 21 on govinfo.gov; View Title 21 Section 820.30 PDF; These links go to the official, published CFR, which is updated annually. As a result, it may not include the most recent changes applied to the CFR. You can learn more about the process here.
21 Cfr Part 820 Pdf
For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 820.1 - Scope. § 820.3 - Definitions. § 820.5 - Quality system. § 820.20 - Management responsibility. § 820.22 - Quality audit. § 820.25 - Personnel. § 820.30 - Design controls. § 820.40 - Document controls. Electronic Code of Federal Regulations (e-CFR) Title 21 - Food and Drugs; CHAPTER I - FOOD AND DRUG ADMINISTRATION, DEPARTMENT OF HEALTH AND HUMAN SERVICES; SUBCHAPTER H - MEDICAL DEVICES; PART 820 - QUALITY SYSTEM REGULATION; Subpart H - Acceptance Activities § 820.80 Receiving, in-process, and finished device acceptance.

